What is Electricity?
Going Atomic
To understand the fundamentals of electricity, we need to begin by focusing in on atoms, one of the basic building blocks of life and matter. Atoms exist in over a hundred different forms as chemical elements like hydrogen, carbon, oxygen, and copper. Atoms of many types can combine to make molecules, which build the matter we can physically see and touch.
Atoms are tiny, stretching at a max to about 300 picometers long (that's 3x10-10 or 0.0000000003 meters). A copper penny (if it actually were made of 100% copper) would have 3.2x1022 atoms (32,000,000,000,000,000,000,000 atoms) of copper inside it.
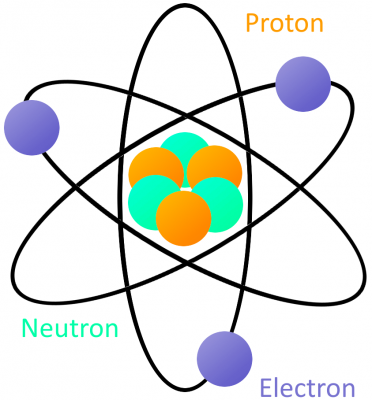
Even the atom isn't small enough to explain the workings of electricity. We need to dive down one more level and look in on the building blocks of atoms: protons, neutrons, and electrons.
Building Blocks of Atoms
An atom is built with a combination of three distinct particles: electrons, protons, and neutrons. Each atom has a center nucleus, where the protons and neutrons are densely packed together. Surrounding the nucleus are a group of orbiting electrons.
Every atom must have at least one proton in it. The number of protons in an atom is important, because it defines what chemical element the atom represents. For example, an atom with just one proton is hydrogen, an atom with 29 protons is copper, and an atom with 94 protons is plutonium. This count of protons is called the atom's atomic number.
The proton's nucleus-partner, neutrons, serve an important purpose; they keep the protons in the nucleus and determine the isotope of an atom. They're not critical to our understanding of electricity, so let's not worry about them for this tutorial.
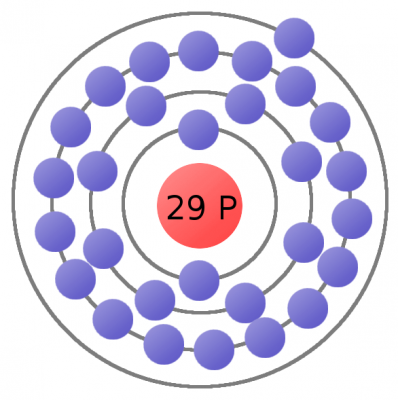
Electrons are critical to the workings of electricity (notice a common theme in their names?) In its most stable, balanced state, an atom will have the same number of electrons as protons. As in the Bohr atom model below, a nucleus with 29 protons (making it a copper atom) is surrounded by an equal number of electrons.
The atom's electrons aren't all forever bound to the atom. The electrons on the outer orbit of the atom are called valence electrons. With enough outside force, a valence electron can escape orbit of the atom and become free. Free electrons allow us to move charge, which is what electricity is all about. Speaking of charge...