What is a Battery?
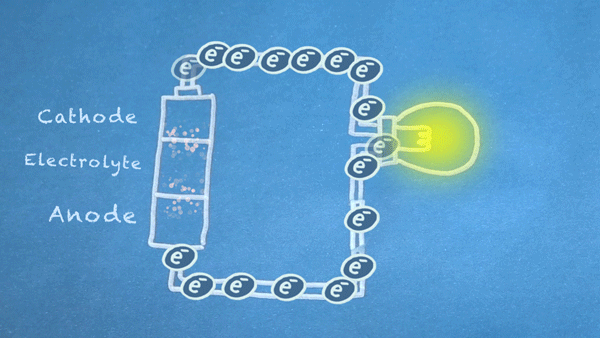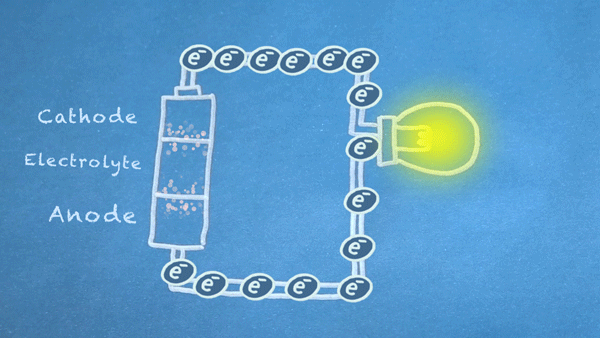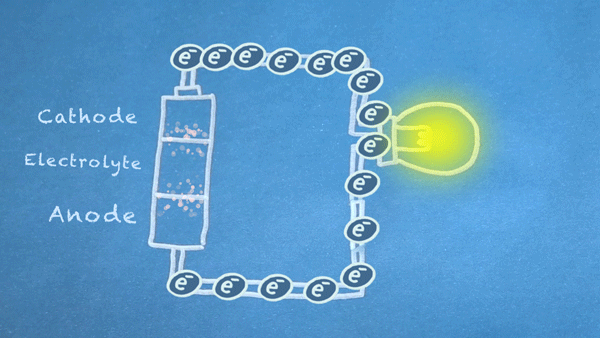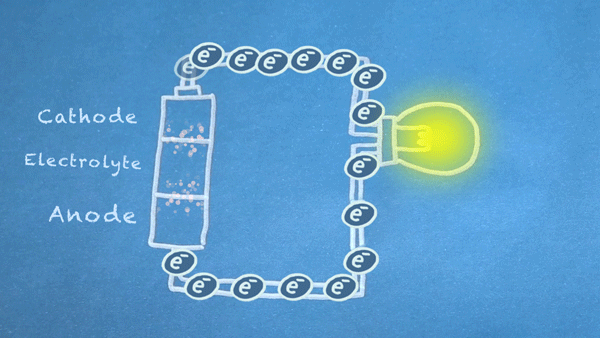
Operation
Batteries generally require several chemical reactions in order to operate. At least one reaction occurs in or around the anode and one or more reactions occur in or around the cathode. In all cases, the reaction at the anode produces extra electrons in a process called oxidation, and the reaction at the cathode uses the extra electrons during a process known as reduction.
In essence, we are separating a certain kind of chemical reaction, a reduction-oxidation reaction or redox reaction, into two separate parts. Redox reactions occur when electrons are transferred between chemicals. We can harness the movement of electrons in this reaction to flow outside the battery to power our circuit.
Anode Oxidation
This first part of the redox reaction, oxidation, occurs between the anode and electrolyte, and it produces electrons (marked as e-).
Some oxidation reactions produce ions, such as in a lithium-ion battery. In other chemistries, the reaction consumes ions, like in the common alkaline battery. In either case, ions are able to flow freely through the electrolyte where electrons cannot.
Cathode Reduction
The other half of the redox reaction, reduction, occurs in or near the cathode. Electrons produced by the oxidation reaction are consumed during reduction.
In some cases, like lithium-ion batteries, positively charged lithium ions produced during the oxidation reaction are consumed during reduction. In other cases, like alkaline batteries, negatively charged ions are produced during reduction.
Electron Flow
In most batteries, some or all of the chemical reactions can occur even when the battery is not connected to a circuit. These reactions can impact a battery's shelf life.
For the most part, the reactions will only occur at full force when an electrically conductive circuit is completed between the anode and cathode. The less resistance between the anode and cathode, the more electrons are allowed to flow, and the quicker the chemical reactions occur.
We can pass these moving electrons through various electrical components, known as a "load," in order to accomplish something useful. In the motion graphic at the beginning of this section, we are lighting a virtual light bulb with our moving electrons.
Dead Battery
The chemicals in the battery will ultimately reach a state of equilibrium. In this state, the chemicals will no longer have a tendency to react, and as a result, the battery will not generate any more electric current. At this point, the battery is considered "dead."
Primary cells must be disposed when the battery is dead. Secondary cells can be recharged, and this is accomplished by applying a reverse electric current through the battery. Recharging occurs when the chemicals perform another series of reactions to take them back to their original state.