What is a Battery?
Components
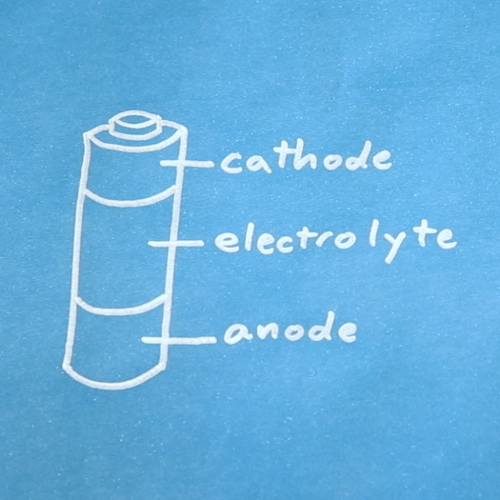
Batteries are made up of three basic components: an anode, a cathode, and an electrolyte. A separator is often used to prevent the anode and cathode from touching, if the electrolyte is not sufficient. In order to store these components, batteries usually have some kind of casing.
Both the anode and cathode are types of electrodes. Electrodes are conductors through which electricity enters or leaves a component in a circuit.
Anode
Electrons flow out from the anode in a device connected to a circuit. This means that conventional "current" flows into an anode.
In a battery, the chemical reaction between the anode and electrolyte causes a build up of electrons in the anode. These electrons want to move to the cathode, but cannot pass through the electrolyte or separator.
Cathode
Electrons flow into the cathode in a device connected to a circuit. This means that conventional "current" flows out from a cathode.
In batteries, the chemical reaction in or around the cathode uses the electrons produced in the anode. The only way for the electrons to get to the cathode is through a circuit, external to the battery.
Electrolyte
The electrolyte is the substance, often a liquid or gel, that is capable of transporting ions between the chemical reactions that happen at the anode and cathode. The electrolyte also inhibits the flow of electrons between the anode and cathode so that the electrons more easily flow through the external circuit rather than through the electrolyte.
-> Alkaline batteries can leak their electrolyte, potassium hydroxide, if subjected to high heat or reverse voltage
(Image courtesy of Wiliam Davies of Wikimedia Commons) <-
The electrolyte is crucial in the operation of a battery. Because electrons cannot pass through it, they are forced to travel through electrical conductors in the form of a circuit that connect the anode to the cathode.
Separator
Separators are porous materials that prevent the anode and cathode from touching, which would cause a short circuit in the battery. Separators can be made from a variety of materials, including cotton, nylon, polyester, cardboard, and synthetic polymer films. Separators do not chemically react with either the anode, cathode, or electrolyte.
Ions in the electrolyte can be positively charged, negatively charged, and can come in a variety of sizes. Special separators can be manufactured that allow some ions to pass but not others.
Casing
Most batteries need a way to contain their chemical components. Casings, otherwise known as "housings" or "shells," are simply mechanical structures meant to hold the battery's internals.
Battery casings can be made of almost anything: plastic, steel, soft polymer laminate pouches, and so on. Some batteries use a conducting steel casing that is electrically connected to one of the electrodes. In the case of the common AA alkaline cell, the steel casing is connected to the cathode.